“The study therefore paves the way for solution-processed conductive materials, which could potentially lead to next-generation organic semiconductors,” according to the University. “Furthermore, the researchers will focus on refining molecular designs to optimise charge transport properties and explore applications in electronic circuits, sensors and energy storage.”
Low solubility of expanded π-electronic systems, used in organic semiconductors, is often a challenge in fabricating assembled structures for organic electronic materials.
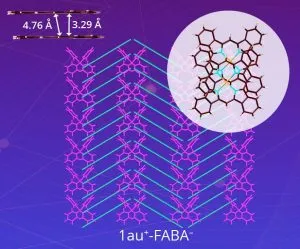
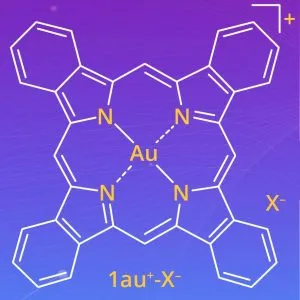
A team from Ritsumeikan University, led by Professor Hiromitsu Maeda, set out to design a soluble version. “We enhanced the solubility of expanded π-electronic cations by combining them with appropriate bulky counter-anions,” he said. Called ‘benzoporphyrin gold complexes’, the materials pair gold ions with various anions (‘X–‘ in the diagram) including: PF6–, FABA–, BArF– and a charged molecule dubbed PCCp–, which led to the generation of ‘less-crystalline’, more soluble states.
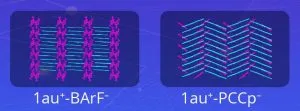
“Both types of structures exhibited electrical conductivity with tunable conductive properties, allowing their use in a range of applications,” said Maeda. “Our study demonstrates new aspects of molecular assemblies and their functionalities through molecular design and synthesis, which are essential for the future applications of π-electronic materials.”
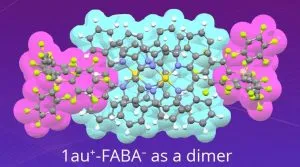
For those who want to understand this, the Chemical Science paper ‘Electrically conductive charge-segregated pseudo-polymorphs comprising highly planar expanded π-electronic cations’ can be read in full without payment.
Middle diagrams above: gold ions in cyan, anions in magenta
Image credit: Hiromitsu Maeda, Ritsumeikan University, Japan
 Electronics Weekly
Electronics Weekly