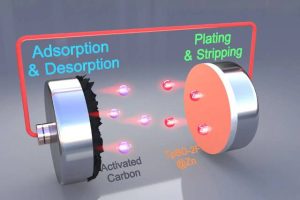
However, as zinc-ions electroplate onto and off of the anode, several destructive processes can occur, including the growth off needle-like ‘dendrites’ (think tin whiskers) and corrosion of the zinc by water – and some of these corrosive reactions release the explosive gas hydrogen.
Now researchers at the Technical University of Munich (TUM) have designed a specific material that cures both dendrite formation and anode corrosion.
It is a form of ‘covalent organic framework’ (COF), which is a class of polymer already used in zinc-ion battery anode research.
But this COF, crafted by PhD student Da Lei, and named ‘TpBD-2F’, is unique in its combination of characteristics.
Firstly, it self-assembles from precursor chemicals on the surface of metallic zinc, thoroughly coating every spot.
And it automatically coats in a even way, that can be predictably stopped – 100μm has been chosen to provide a layer thin enough to be highly porous to Zn2+ ions, but thick enough to block water access.
Its porosity comes from having a honeycomb-like crystal structure that grows from the metal surface, leaving hexagonal channels all the way from the surface to the outside world.
Having many identical parallel pores of similar length causes zinc ions to plate evenly on the zinc surface, discouraging dendrite growth.
Fluorine atoms have been designed into the polymer crystal lattice for two purposes: they repel water, and that provide sites for zinc ions to hop between facilitating travel along the pores.
This dual action is particularly ingenious because zinc often drifts in aqueous solution as a hydrated ion (Zn(H2O)6)2+), which gets both transported and dried by the fluorinated pore.
“Zinc-ion batteries with this protective layer could replace lithium-ion batteries in large-scale energy storage applications, such as in combination with solar or wind power plants,” said Da.
The anode has been tested in a button cell zinc-ion capacitor – a structure that has a capacitive cathode but the same anode as a zinc-ion battery – in which a lifetime of 100,000 cycles was demonstrated charging and discharging at 5A/g.
“This is truly a spectacular research result, said project head Professor Roland Fischer. “The chemical approach developed by Da Lei not only works but is also controllable. As fundamental researchers, we are primarily interested in new scientific principles—and here we have discovered one. I see no reason why our findings couldn’t be translated to larger applications, now it’s up to engineers to develop appropriate production processes.”
For further information, the whole of ‘Ion-transport kinetics and interface stability augmentation of zinc anodes based on fluorinated covalent organic framework thin films’ can be read without payment in Advanced Energy Materials
 Electronics Weekly
Electronics Weekly